1. Introduction
While it has been over a decade since 193 countries committed to the United Nations 2030 Agenda, the world energy matrix is still highly dependent on fossil fuels. To ensure sustainable development and mitigate global warming, biofuel adoption will need to replace gasoline and diesel use. Producing ethanol from algae biomass (third-generation or 3G ethanol) meets not only the Sustainable Development Goals (SDGs) of the 2030 Agenda but also the Paris Agreement on the reduction of greenhouse gas (GHG) emissions, which was recently ratified during the 26th United Nations Conference on Climate Change (COP26), in Glasgow, Scotland.
Currently, 3G ethanol is primarily a prospective technology of the future [1,2], as second-generation ethanol is only in the initial steps of full-scale commercial production [3], [4], [5]. Still, researchers worldwide have recently begun exploring methods to optimize 3G ethanol production. When searching the literature for the terms "alg* ethanol" OR "3G ethanol" OR "third-generation ethanol", the Scopus database (www.scopus.com) shows 52 documents from 1958 to 2022; of these, 27 were published in the last five years. Some of these studies have focused on improving algal biomass production, its carbohydrate content, and/or the sugar extraction efficiency (since sugars are indispensable for alcoholic fermentation) [6], [7], [8], [9], [10], while others show the potential use of wastewater and agro-industrial residues [11,12] and the possibility of combining and diversifying algal species [13,14].
In contrast, even though the options for algal biomasses are varied and the technology has proven feasible, algae-ethanol production faces bottlenecks that need to be addressed to make it more economically attractive, notably in the fermentation stage. Currently, the yeast most widely employed in the fuel ethanol industry (Saccharomyces cerevisiae) cannot ferment many of the sugars available in algae hydrolysates, thus decreasing fermentation efficiency and ethanol yield [15,16]. To circumvent this issue, new wild yeasts are being prospected, and the well-established S. cerevisiae has been engineered to allow the conversion of algae carbohydrates into the desired biofuel [17], [18], [19], [20], [21], [22].
In this analysis of the production viability of 3G ethanol, the following sections will address: (i) the main biochemical features of different algae for bioethanol production; (ii) the state of the art in cultivation, harvesting, and biomass pretreatment and saccharification; (iii) recent strategies to improve the fermentation phase; (iv) ways of decreasing water footprint in the ethanol production; and (v) a comprehensive evaluation of mass and energy balances during the process.
2. Algae biomass as feedstock for bioethanol production
Algae are photosynthetic organisms usually divided into macroalgae and microalgae based on their morphology and size, ranging from micrometers up to 70 m [23,24]. Macroalgae can be classified into three groups: brown seaweeds (Phaeophyceae), red seaweeds (Rhodophyceae), and green seaweeds (Chlorophyceae); whereas microalgae are generally grouped into diatoms (Bacillariophyceae), green algae (Chlorophyceae), golden algae (Chrysophyceae), and cyanobacteria (Cyanophyceae) [23].
Macroalgae are found primarily in marine environments, while microalgae species can be found in both freshwater and marine environments [24]. Since algae synthesize large amounts of carbohydrates as energy storage, their biomass can be used to produce bioethanol, a renewable fuel [25,26]. The carbohydrate content of some algae species is shown in Table 1, illustrating the difference between each classification and even between species from the same genus.
Table 1. Carbohydrate content from different species of macroalgae and microalgae.
| Species | Carbohydrates (%)* | Additional information | Reference |
|---|---|---|---|
| Macroalgae | |||
| Gracilaria sp. | 76.7 | The red alga was obtained from a market in Taiwan, China | [27] |
| Gracilaria sp. | 56.3 | The red and brown seaweeds were collected from Mandapam coastal regions, India | [28] |
| Sargassum sp. | 45.4 | ||
| Sargassum fulvellum | 39.6 | These fresh brown algae were purchased from a seaweed market in South Korea | [29] |
| Laminaria japônica | 51.9 | ||
| Gelidium amansii | 77.2 | The dried red and green algae, respectively, were obtained from a market in South Korea | |
| Ulva lactuca | 54.3 | ||
| Ulva pertusa | 59.1 | The green, brown and red seaweed, respectively, were obtained from a supermarket in Japan | [30] |
| Laminaria japônica | 54.5 | ||
| Gelidium amansii | 71.4 | ||
| Microalgae | |||
| Dunaliella salina | 50.6 | High light intensity, nitrogen-limited, and 10% CO2 | [31] |
| Dunaliella sp. | 29.6 | Cultivation with 10 ppt salinity and harvesting in stationary phase | [32] |
| Scenedesmus sp. CCNM 1028 | 43.4 | 21 days of nitrogen starvation | [33] |
| Scenedesmus obliquusCNW-N | 51.8 | 3-day nitrogen starvation under 140 µmol m−2 s−1 of light intensity, and 2.5% of CO2 feeding | [34] |
| Anabaena variabilis | 63.4 | Cultivation with biphasic phosphate-starved conditions | [35] |
| Microcystis aeruginosa | 55.1 | ||
| Chlamydomonas reindhardtii UTEX 90 and CC 2656 | 52.2 and 45.0 |
Mixotrophic cultivation, conical flask (250 mL), 23°C, photoperiod of 14 h, illumination of 100 μmol m−2 s−1, 100 rpm |
[36] |
| Chlorella vulgaris FSP-E | 51.3 | 4-day nitrogen starvation under 450 µmol m−2 s−1 of light intensity, and 2% of CO2 aeration | [37] |
| Chlorella sp. AE10 | 75.9 | Cultivation with phosphorus starvation conditions using red LED 850 mmol m−2 s−1 | [38] |
| Chlorella homosphaera | 54.0 | Highest carbohydrates content was obtained with 50% less nitrogen and 20% more NaCl | [39] |
| Spirulina platensis LEB 52 | 65.5 | ||
| Spirulina sp. LEB 18 | 69.8 | Cultivation in aquaculture wastewater supplemented with Zarrouk 25% | [40] |
| Spirulina sp. LEB 18 | 63.3 | Cultivation with 0.25 g L−1 of NaNO3 and CO2 addition for 1 min with 120 ppm of fly ashes | [41] |
| Spirulina platensis LEB 52 | 72.0 | Cultivation performed in mini-open raceways (10 L) with 0.35 m s−1 of agitation speed | [42] |
*dry weight.
Carbohydrate content varies between macro- and microalgae, and also between genera and species within each classification. Red seaweeds, with their carbohydrate content of 56.3-77.2% (Table 1), seem to be the best option to produce bioethanol from macroalgae biomass. However, Becker [43] reports the predominance of proteins among the accumulated compounds by microalgae. As shown in Table 1, some microalgae species can accumulate a high level of carbohydrates, especially under cultivation conditions with environmental and nutritional stress. Biomass of several microalgae strains can be composed of more than 50% carbohydrates, achieving >70% under certain culture conditions (Table 1), which emphasizes that the steps of strain selection and cultivation mode are essential for the aimed application of microalgae. Chlamydomonas, Chlorella, and Synechocystis have been the main genera of microalgae for biofuel production [44]. Besides bioethanol production, the carbohydrate content of algae biomass is important for producing other biofuels, such as biohydrogen and biomethane, which illustrate the great potential of algae for bioenergypurposes.
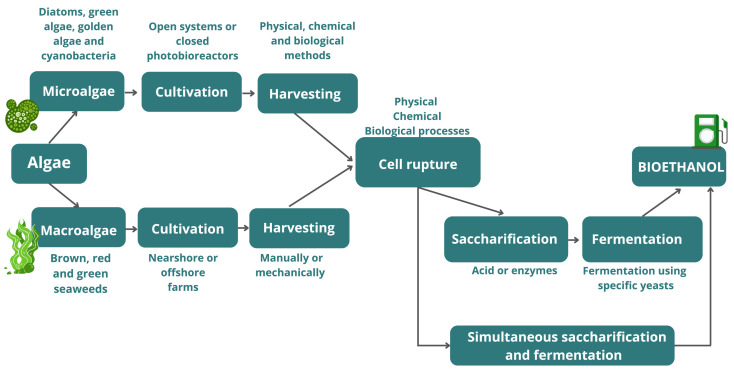
In addition to different chemical compositions, some aspects of bioethanol production are specific to macro or microalgal biomasses, as shown in Fig. 1, resulting in advantages and disadvantages between these two matrices, as presented in the following subitems (2.1-2.5).
 Fig. 1. Schematic diagram of the bioethanol production from algae biomass.
Fig. 1. Schematic diagram of the bioethanol production from algae biomass.2.1. Cultivation
There are significant differences in methods for cultivating macro- and microalgae, making the process specific to each of these organisms [24]. The first step for a variety of uses (including bioenergy production) is biomass generation [45]. In the case of macroalgae, Laminaria japonica and Undaria pinnatifida (brown algae), and Eucheuma spp., Kappaphycus alvarezii, and Gracilaria verrucosa (red algae) are the most frequently cultivated species [46].
Macroalgal cultivation can be carried out in seawater using two systems. Nearshore farms are the most widely used (about 90% of the seaweed production), as they can be built near shorelines with shallow depths. In the offshore method, farms are constructed in deep waters to avoid the influence of the land, which requires growth structures anchored to the ocean bottom or floating lines with positioning devices [45,47].
Macroalgae are commonly cultivated with farming technology, and grown using kelp systems aligned vertically or horizontally [45,46]. In the vertical method, the rope is held vertically by weights placed at one end, while in the horizontal method, floating lines are connected by horizontal ropes [45]. Since carbohydrate compounds are abundant in macroalgae biomass, these organisms are considered promising sources for bioenergy applications, especially for bioethanol production [46,48].
Microalgae biomass has also been reported as a promising feedstock for biofuel uses [49]. Different conditions in microalgae cultivation result in significant changes in the concentration, productivity, and biochemical composition of biomass [50]. Therefore, the selection of the correct cultivation method is essential to ensure the subsequent use of the biomass [51,52]. In addition, microalgae cultures must consider design, cost, contamination risks, and cleaning, especially for commercial purposes [49]. Strain type and nutrient source also impact the cultivation method selection [50].
To increase the feasibility of using microalgae for bioethanol production, the carbohydrate content and biomass productivity need to be improved, which can be achieved through changes in different cultivation parameters [53,54]. This is possible due to the ability of microalgae to modify their biochemical composition in response to culture manipulation, which includes environmental and nutritional changes in the cultivation [55]. Light intensity, pH, salinity, and temperature are the main environmental factors that influence the biochemical composition of microalgae, while nutritional factors include the availability and type of source of nitrogen, carbon, phosphorus, sulfur, and iron [56], [57], [58]. Microalgal engineering (e.g. genomic, transcriptomic, proteomic, metabolomic strategies) is another promising alternative to enhance biofuel production, especially by increasing the productivity of biomass and carbohydrate accumulation of several microalgae strains [59], [60], [61]. These techniques can improve stress tolerance and photosynthetic rate, which directly impact biofuel production viability [62].
Microalgal cultivation can be classified as autotrophic, mixotrophic, or heterotrophic, with autotrophic being the most used method [52,54]. In this type of cultivation, the carbon source is inorganic, and light serves as a source of energy. In mixotrophic cultivation, both organic and inorganic carbon sources can be used. In heterotrophic cultivations, organic carbon serves as energy and a carbon source [54]. There are three main operational modes for growing microalgae: batch, semicontinuous or continuous [63].
Regarding the accumulation of carbohydrates, de Farias Silva et al. [64]performed a comparison between batch and continuous modes. The batch system can be divided into two stages: in the first, the biomass grows in an environment with excess nutrients, while in the second, the carbon is converted into energy reserves, causing the accumulation of carbohydrates and lipids. In this second stage, biomass growth almost stopped, and although carbohydrate productivity increased, biomass productivity decreased from 40% to 60%. By using a lower nitrogen concentration in continuous mode, it is possible to achieve growth and accumulation of carbohydrates simultaneously, reaching a stationary phase with carbohydrate productivity two to three times higher [64].
Microalgal biomass production can occur through open systems or closed photobioreactors [65]. Open ponds are more widely applied on a commercial scale due to economic and operational aspects but have low biomass productivity compared to closed photobioreactors. This high productivity is due to this system's greater control of cultivation variables [66]. According to Suparmaniam et al. [49], a raceway pond is the best option to cultivate microalgae for commercial biofuel production, whereas closed photobioreactors are more suitable for producing microalgae to obtain biomass with higher-value products. While macroalgae are mainly cultivated in coastal regions, microalgae cultures have the advantage of not requiring arable land and can use wastewater as a nutrient source, reducing cultivation costs.
2.2. Harvesting
After algae cultivation, macro or microalgae biomasses are harvested for further processing and applications [50]. Macroalgae have characteristics similar to plants and, as they are larger than microalgae, their harvest is technologically and economically advantageous [67], [68], [69]. Macroalgae can be harvested manually or mechanically. In the manual method (the most common), algae are harvested through a sickle, fork, or net, while mechanized methods require harvesters in boats or ships, such as rotation blades, suction, or dredging cutters [45].
The primary issue in the harvesting stage is separating a small amount of microalgae biomass from a large volume of culture medium [70]. The size of the microalgae cells (generally <20 µm), combined with high colloidal stability and a density slightly higher than that of the water, results in the spontaneous non-sedimentation of the microalgae cells by gravity [70,71]. These characteristics make harvesting one of the costliest steps in producing microalgae [72]. Mata et al. [73] report that harvesting microalgae can represent 20-30% of total biomass production costs. Therefore, harvesting is identified as a bottleneck for microalgae commercialization [49].
Given this bottleneck, several alternative strategies have been developed to optimize microalgae harvest to achieve a better cost-benefit ratio [70,74]. Microalgal harvesting can be performed using mechanical, electrical, biological, and chemical methods, which are generally divided into thickening (coagulation/flocculation, bioflocculation, gravity sedimentation, flotation, and electricity-based methods) and dewatering (centrifugation and filtration) [75]. Each microalgae harvesting method presents advantages and disadvantages related to biomass recovery rate, costs, and execution parameters [72,[75], [76], [77], [78]]. Physical methods are commonly reported as demanding high energy costs while using chemical products to harvest microalgae can make the subsequent use of biomass unfeasible due to heavy metal contamination [79]. As an alternative, the interest in using bioflocculants to harvest microalgae has been growing, since they are considered non-toxic, more sustainable, safe-handling, and eco-friendly [49,80]. Therefore, the harvest of macroalgae biomass is easier than microalgae since they can be harvested manually because of their size.
2.3. Cell rupture
To transform the biomass of microalgae and macroalgae into bioethanol, it is necessary to first rupture the cell wall, in order to release the intracellular compounds of interest, which, in the case of bioethanol, are carbohydrates [81,82]. Each species has a specific cell wall composition, which determines cell viability in a wide range of environments, defending cells from biotic and abiotic stresses and providing plasticity, which allows different cells to expand and form [83,84].
The cell wall structures of microalgae may differ in the functions of the growing environments and between species. To extract more intracellular organic compounds from microalgae, it is necessary to destroy the protective barrier of the cell wall. A microalgae cell wall is composed of mannans, glucans, chitinpolysaccharides, arabinogalactans, and rhamnose, which can be found through different types of glycoside visualizations, and extracted and quantified as carbohydrates [26,85,86].
The cell wall of microalgae also has proteins and clusters of amino acids, such as valine, alanine, glutamic acid, and glycine. This protein matrix adds structural integrity to the cell wall. In addition, the cell wall of the microalgae has lipid contents, basically formed by palmitic acid, stearic acid, and 1,3-di-tert-butylbenzene. Other elements make up the structure of the cell wall and its layers; these layers are called microfibrillaries, which form the rigid part of the wall. It is noteworthy that the cell wall of microalgae can change significantly under different environmental conditions, such as nutrient depletion [26,86].
In general, macroalgae (primarily marine lineages) have cell walls formed by sulfated polysaccharides, rich in mannose, xylose, arabinose, and glucose, in addition to xylan, mannan, and glucan. They also have protein and lipid structures very similar to the microalgae wall. Macroalgae have a higher amount of these sulfated polysaccharides, which form macromolecules that promote flexibility and resilience. They act against physical forces exerted by waves and ocean currents, preventing their drying out when exposed to high solar radiation and stress when exposed to variations in salinity and pH [26,87,88].
Due to the similarities between the microalgae and macroalgae cell walls and the variability between species, there are no specific cell disruption methods for each alga; both have thick cell walls with very similar compositions. The most frequently used methods are physical (ultrasound, high-pressure homogenization), chemical (acid and base solutions), and biological processes(enzymes) [89], [90], [91].
However, it is worth mentioning that some macroalgal species may have more complex structures in their intracellular compounds, such as sulfated polysaccharides, which would require more complex pretreatments or even the use of high temperatures and pressure. These more complex pretreatments are a disadvantage to macroalgae cultivation because, in addition to requiring greater investments, they can lead to the formation of by-products that would alter the final ethanol yield [92], [93], [94].
2.4. Saccharification of polysaccharides
After releasing algal intracellular compounds, they must be hydrolyzed to be transformed into different monosaccharides for the later fermentation stage. Saccharification strategies must prioritize high efficiency and reduction of generation of by-products that can influence fermentation [95], [96], [97].
A series of methodologies are employed to transform intracellular carbohydrates from microalgae, using concentrated acids and commercial enzymes. According to the literature, these processes can be developed simultaneously to optimize the saccharification process. However, depending on the microalgae species, optimization can be achieved using only enzymatic hydrolysis methods. Enzymatic hydrolysis is widely used for providing milder pH and temperature conditions and less by-product formation. Two enzyme complexes are used to obtain complete hydrolysis of the microalgal polysaccharides: amylases (alpha-amylase and amyloglucosidase) and cellulases (endoglucanases, exoglucanases, beta-glucosidases). Thus, the hydrolysis of microalgae compounds can be expensive, in addition to the use of different processes, which makes large-scale implementation difficult [26,82,98].
During the hydrolysis of macroalgal polysaccharides, intracellular compounds such as starch and cellulose are transformed into fermentable sugars. The same difficulties of large-scale implementation apply to the production of macroalgal biomass, as they share a similar process. Like macroalgae, a set of specific enzymes must be used in the hydrolysis of microalgae, which can complicate the process, especially in large-scale implementation. However, macroalgae have an advantage over microalgae, as they can accumulate an average value of 60% of carbohydrates, which increases the yield of reducing sugar formation, making the process more advantageous [99,100].
Macroalgae contain particular polysaccharides and monosaccharides, such as ulvan, fucoidan, alginate, laminaran, floridian starch, mannitol (a sweetened alcohol), rhamnose, fucose, and uronic acids. In some algae, these compounds can be grouped in the form of agar, characterized as a polymer of galactose and galactopyranose, which is transformed into reducing sugars when hydrolyzed. Some of these polysaccharides are not present in microalgae so higher yields can be obtained after the hydrolysis of macroalgae [97,101]. Still, some species of macroalgae contain sulfated polysaccharides, generating compounds that can interfere with subsequent fermentation routes and impair ethanol production yields [92], [93], [94].
2.5. Fermentation
After hydrolysis, the monomers must be fermented to be transformed into bioethanol or another product of interest. Two methodological processes can produce ethanol from microalgae: fermentation using specific yeasts that use reducing sugars from microalgae biomass and direct production by genetically modified microalgae. The polysaccharides extracted from the microalgae can be fermented into ethanol through fermentation routes very similar to the consolidated production of starch cultures. This is an advantage of microalgae, since the fermentative routes are already established, helping implement these processes on large scales [26,81,96,102].
There are two types of yeast fermentation: simultaneous saccharification or separated saccharification and fermentation [103]. In the case of separated saccharification and fermentation, the pretreated microalgal biomass is hydrolyzed to glucose and subsequently fermented to bioethanol in separated units, while simultaneous saccharification and fermentation occur in a single step [104]. Saccharification and separate fermentation have the following advantages: low cost for chemicals, short duration, and simplicity for large-scale application. On the other hand, simultaneous saccharification and fermentation has fewer steps and higher bioethanol yields [104]. Yeasts of the genus Saccharomyces or bacteria of the genus Zymomonas are generally used in these processes [56]. Since ancient times, S. cerevisiae has been used in biotechnology to produce alcoholic beverages, as it has high efficiency in converting sugars, mainly glucose, into ethanol [104]. Per Costa and De Morais [105], microalgae are a potential source of fermentable substrates and may have high levels of carbon compounds in their composition, which can be fermented directly or after pretreatment. However, they also report that other compounds, such as CO2 and H2O, are generated during bioethanol production. Therefore, the maximum theoretical yield is 0.51 kg of ethanol and 0.49 kg of CO2 per kg of glucose.
The same processes can be used to ferment macroalgal monosaccharides. The difference is that macroalgae have particular monosaccharides that are fermented by specific routes, which require the use of specific biocatalysts, resulting in greater cost, which may render the process unfeasible. Many macroalgae have fractions of mannitol and laminaran, and these extracts are removed and fermented by specific microorganisms [46,106]. Such specific monosaccharide fractions are not present in microalgae, which adds an advantage to macroalgae. However, it is still necessary to optimize processes that carry out the total fermentation of macroalgal carbohydrate fractions (alginate, laminarin, and mannitol) to be converted into ethanol with high yields and productivity. This optimization of total fermentation can be achieved through the development of a microorganism capable of acting on all fractions of substrates. Nevertheless, such optimization still lacks technological advances, making it difficult to implement large-scale production and meet the biorefinery purpose, that is, the total use of the macroalgal fraction [29,94].
Such process optimization can lead to obtaining ethanol with higher yields or in high concentrations from the strategies listed here, such as changes in cultures, to increasingly obtain biomass of micro or macroalgae with high levels of carbohydrates. This provides the generation of hydrolysates with high levels of reducing sugar, through the use of a set of enzymes capable of acting on all the polysaccharides present in the biomass. After generating these fermentable sugars, the use of different yeasts can increase conversion yields (see the following section). In addition to this, purification strategies can be optimized through distillation to remove water and other impurities in the dilute alcohol product that can reach 10–15% ethanol [107]. Such processes and factors could thus optimize ethanol production (in terms of both concentration and quality) from algal biomasses.
3. Engineering yeasts for algal-carbohydrates fermentation
As mentioned before, after the saccharification of algal biomass, for both macroalgae and microalgae, carbohydrates are available for fermentation; however, the efficiency of the fermentation step is crucial to obtain high ethanol yields. Several studies have evaluated the fermentative potential of yeasts from algal raw material (Table 2). Yeasts are biologically designed to convert carbohydrates into ethanol through alcoholic fermentation and have been widely used for their ability to use several carbon sources [108]. Saccharomycescerevisiae is a well-established yeast in the production of alcohol, such as beer and wine [109], and first-generation fuel ethanol, which has glucose or sucroseas the main substrates [110]. However, the composition of different macro- and microalgae requires organisms capable of fermenting pentoses and other hexoses, as previously described, as well as tolerating a wide pH range, high temperatures, high ethanol concentration, and osmotic stress found in the fermentation wort. Thus, the identification of non-Saccharomyces yeasts with the aforementioned characteristics, along with the genetic engineering of S. cerevisiae, has proven necessary for fermenting a greater diversity of carbohydrates and increasing the efficiency of the process [2].
Table 2. Comparison of bioethanol production by yeasts using macroalgae and microalgae as feedstock.
| Algae feedstock | Yeast species | Saccharification approach | Ethanol (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Macroalgae | |||||
| Ascophyllumnodosumsp. | Schefferomyces stipitisNCYC1542 | Acid/enzyme | 2.4 | 0.16* | [111] |
| Chondruscrispus sp. | Pichia anomala | Acid | 3.3 | 0.24* | [112] |
| Gelidium amansii sp. | Scheffersomyces stipitis | Acid/thermal | 11.5 | 0.34 | [108] |
| Acid/enzyme | 22.0 | 0.50 | [22] | ||
| Gelidium elegans sp. | S. cerevisiaeNBRC 10217 | Acid | 13.27 | 0.30* | [113] |
| Gracilaria gigas sp. | S. cerevisiaeATCC 200062 | Acid/enzyme | 3.56 | 0.15 | [114] |
| Gracilaria verrucosa | S. cerevisiaeHAU | Enzyme | 14.89 | 0.43 | [92] |
| Kluyveromyces marxianus | Acid/enzyme | 29.0 | 0.50 | [115] | |
| Laminaria digitata | S. cerevisiaeNCYC2592 | Acid/enzyme | 3.0 | 0.48* | [116] |
| Kluyveromyces marxianusNCYC1424 | Acid/enzyme | 6.0 | 0.20* | [111] | |
| Saccharina latissima | S. cerevisiae | Enzyme | 12.83 | 0.42 | [117] |
| Pterocladiella capillacea | Kluyveromyces marxianus | Acid | 10.6 | 0.39 | [118] |
| Ulva fascina | S. cerevisiae | Acetate buffer/enzyme | 1.35 | 0.45 | [119] |
| Ulva lactuca | Pichia anomala | Acid | 3.5 | 0.30* | [112] |
| Microalgae | |||||
| ArthrospiraplatensisNIES39 | S. cerevisiaeMT8-1dGS | Enzyme | 48.0 | 0.27 | [120] |
| Chlamydomonas mexicana YSL008 | S. cerevisiaeYPH499 | Sonication/enzyme | 10.5 | 0.50 | [121] |
| Desmodesmus sp. | S. cerevisiae | Acid/lyophilization | 61.2 | 0.31 | [122] |
| Scenedesmus obliquus | Kluyveromyces marxianusIGC2671 | Acid | 11.7 | 0.28* | [123] |
| Saccharomyces carlsbergensisATCC 6269 | Acid | 11.2 | 0.27* | ||
| Synechococcus sp.PCC7002 | S. cerevisiaeThermosacc® Dry | Sonication/enzyme | 30.0 | 0.27 | [124] |
*Calculated values with the data available in the work as Yield = ethanol produced/sugar supplied.
Red algae, for example, have galactan (carrageenan and agar) as the main polysaccharide, which consists of units of D-galactose and 3,6-anhydro-galactose [125], and it is known that galactose consumption is inhibited by glucose repression [126]. Thus, some studies have found that yeasts previously adapted to a high concentration of galactose allow a more efficient fermentation of this sugar and eliminate the repression exerted by glucose. Hargreaves et al. [127] fermented K. alvarezii biomass and observed an increase in ethanol production when the yeast S. cerevisiae CBS1782 was pregrown in a synthetic medium containing galactose. Similarly, the adaptation of the yeasts Kluyveromyces marxianus KCTC7150 and Candida lusitaniae ATCC42720 to galactose allowed the simultaneous consumption of glucose and galactose from the same biomass. Those yeasts showed, respectively, ethanol yields of 0.31 g/g (K. marxianus) and 0.37 g/g (C. lusitaniae), with consumption of 81% and 86% of galactose after 144 h incubation [128]. An even greater advantage was observed for Scheffersomyces (Pichia) stipitis pregrown in high-concentrated galactose media, which yielded 0.5 g of ethanol per g of fermentable sugar from a red seaweed Gelidium amansii hydrolysate [22].
Using CRISPR/Cas-9, Sukwong et al. [18] simultaneously deleted three glucose-mediated repressor genes (GLK1, MIG1, and MIG2) and overexpressed a phosphoglucomutase (PGM2) in a laboratory S. cerevisiae strain. This strategy improved the yeast sugar consumption rate sixfold and made it reach a fermentation efficiency of 90% (compared to the theoretical maximum) in hydrolysates of the red seaweed G. verrucosa. Also, through genetic engineering, Lee et al. [129] verified that the construction of the mutant S. cerevisiae HJ7-14, resistant to 2-deoxy-D-glucose, allowed moderate relief from the glucose-mediated repression and a higher bioethanol production capacity. The authors found that, in 12-hour batch fermentation, HJ7-14 produced 7.4 g/L of ethanol from hydrolysates of the red alga G. amansii, which was 50% faster than that observed for the parental strain.
Taking two algae of similar carbohydrate composition (the red alga Gracilariasp., 56%, and the brown seaweed Sargassum sp., 45%), Saravanan et al. [28]found a reduction of 141 and 110 mg, respectively, of sugar per gram of biomass after acid/enzymatic hydrolysis. According to the authors, fermentation with Hanseniaspora opuntiae GK01 yielded 27 g/L and 18 g/L of ethanol production from Gracilaria sp. and Sargassum sp., respectively, which was similar to the fermentation carried out by S. cerevisiae (29 and 20 g/L). The lower yield from brown algae has been attributed to its complex composition, considering the presence of alginate, mannitol, and glucan [130].
To overcome the above-mentioned bottleneck, Takagi et al. [131] constructed an alginate-assimilating S. cerevisiae strain (named Alg1) by overexpressing genes that encode endo- and exo-type alginate lyases, a permease for the alginate monomer 4-deoxy-L-erythro-5-hexoseulose uronic acid (DEHU), and components of the DEHU metabolic pathway. When Alg1 was cultured in alginate for 12 h, the alginate degradation products were predominantly monosaccharides, with smaller amounts of oligosaccharides. Afterward, the authors attempted to increase Alg1 mannitol metabolizing capacity through prolonged cultures of this strain in media containing mannitol as the sole carbon source. The resulting strain AM1 (alginate- and mannitol-assimilating yeast) successfully produced ethanol from alginate and mannitol. Interestingly, the authors found twice as much ethanol production when the yeast was precultured in a medium rich in alginate and mannitol (8.8 g ethanol/L) compared to alginate-free cultivation (4.2 g ethanol/L). Similarly, Sasaki et al. [132] observed that a co-culture of AM1 (alginate- and mannitol-assimilating yeast) with a cellulase-displaying S. cerevisiae strain (CDY) without pre-adaptation in media rich in alginate and/or mannitol resulted in a lower production of ethanol (2.1 g/L) from the biomass of the brown macroalgae Ecklonia kurome (5%, w/v).
For S. cerevisiae to simultaneously and efficiently metabolize alginate and mannitol (two of the most abundant carbohydrates in brown macroalgae), a rigorous adaptation in the cellular redox potential is required. Indeed, at the first reaction of mannitol catabolism, this sugar-alcohol is oxidized into fructose (by mannitol-2-dehydrogenase — M2DH), generating a surplus of reducing equivalents in the form of NADH. Thus, unless a recyclable way of reoxidizing NADH into NAD+ is present, mannitol metabolism may lead the cells to a redox imbalance [133]. Enquist-Newman et al. [134] engineered a S. cerevisiae strain to concomitantly overexpress (i) a DEHU transporter from the alginolytic fungus Asteromyces cruciatus, (ii) bacterial genes responsible for DEHU catabolism, (iii) a mannitol transporter, and (iv) the enzyme M2DH. This is because the first reaction in DEHU catabolic pathway (catalyzed by a DEHU reductase) allows NADH reoxidation into NAD+, thus counterbalancing the excess of reducing equivalents produced from mannitol consumption. After genetic engineering, the authors submitted the engineered strain to a long-term adaptation period in media with DEHU alone or DEHU and mannitol together. They then obtained the first S. cerevisiae strain that fermented these carbon sources into ethanol with up to 83% of the maximum theoretical yield from consumed sugars.
These data demonstrate that the engineering of microorganisms has been an important strategy in ethanol production by combining characteristics that allow greater adaptation to the conditions of the fermentation tank and higher product yield [2,130,[135], [136], [137]].
4. Tolerance to NaCl by fermenting microorganisms, in the hypothesis of use of seawater
As demand increases, concerns arise regarding the process's high water footprint (WF), which refers to an indicator of the amount of freshwater used during the production process [138,139]. For each gallon of ethanol produced from corn, an estimated 3 to 4 gallons of fresh water are needed [140].
Recent studies indicate that an alternative to reducing freshwater use in ethanol production is replacing it with seawater in the fermentation vats [141,142]. The substitution of fresh water with seawater has been evaluated as a method for reducing WF in ethanol production, which may positively impact biorefinery strategies based on this technology. This method would generate more economical, efficient processes with fewer environmental impacts and would reduce the demand for water resources [143], [144], [145], [146], [147], [148], [149].
Over the last two decades, there have been efforts to develop viable technologies for commercial-scale implementation using seawater (Table 3); however, many factors affect this process, mainly due to high salinity, which can vary between 2.5 to 3.5% [143,[147], [148], [149], [150]]. The presence of high salt concentrations can reduce the efficiency or inhibit the fermentative capacity of some microorganisms frequently used for ethanol production due to the high osmotic tension [145,148]. Therefore, exploring salt-tolerant yeasts with an efficient ethanol production capacity is a big step toward producing biofuel from hydrolysates with high salt content [151].
Table 3. Studies based on the substitution of freshwater for seawater using yeasts tolerant to the saline environment.
| Yeasts | Critical inhibitory NaCl concentration (%) | Substrate | Initial sugar concentration (g/L) | Fermentation | Reference | |||
|---|---|---|---|---|---|---|---|---|
| NaCl source | Conditions | Concentration (g/L) | Yield (g/g) | |||||
| Terrestrial Yeasts | ||||||||
| Saccharomyces cerevisiaeNCYC2592 | 6.40 | Glucose (5.5 % w/v) | 55 | Seawater |
30°C 400 rpm 24 h |
25.75 ± 0.58 | N/A | [152] |
| Pichia stipis Y7124 | N/A | Synthetic culture medium | 20 | Seawater |
30°C 250 rpm 11 h |
7.34 ± 0.06 | 0.38 ± 0.01 | [141] |
| Marine Yeasts | ||||||||
| Saccharomyces cerevisiae AZ65 | 13.70 | Sugarcane molasses (30 % w/v) | 138.8 ± 2.37 | Seawater |
30°C 200 rpm 48 h |
52.23 ± 2.19 | N/A | [152] |
| Saccharomyces cerevisiae AZ118 | 14.40 | Glucose (5.5 % w/v) | 55 | Seawater |
30°C 400 rpm 24 h |
23.72 ± 1.16 | N/A | |
| Citeromyces matritensis M37 | 20.00 | Salted wakame pretreated with sulfuric acid (0.3 % v/v) and heat | 6.33 | 15 % NaCl by salted wakame |
25°C 96 h |
2.58 | N/A | [151] |
| Candida sp. | 15.00 | Kappaphycus alvarezii(red algal) pretreated with sulfuric acid (2.5 % v/v) | 33.7 | 9 % NaCl by algal |
30°C 48 h |
17.6 | N/A | [154] |
| Wickerhamomyces anomalus M15 | 10.70 | Ulva linza(green algal) pretreated with sulfuric acid (2.5 % v/v) | 50 | 5% NaCl by algal |
30°C 200 rpm 240 h |
48.2 | 0.329 | [144,159] |
The use of seawater can positively impact the cellular functions of microorganisms due to the presence of compounds other than sodium chloride (NaCl) salt, which can improve the fermentative capacity and induce resistance to osmotic stress [152]. The presence of salts in non-inhibitory concentrations can result in mild osmotic stress, which activates different mechanisms in yeast cells. These mechanisms require energy or carbon, causing an increased glucose consumption rate in non-inhibitory salt concentrations [153].
The yeast's osmotic stress adaptation results from complex cellular adaptation mechanisms that differ between yeast species, integrating their genes, regulatory networks, and signaling pathways. Marine yeasts generally have fermentative characteristics that are more adapted to a seawater environment; they show faster fermentation rates because they present a shorter adaptation phase to the fermentative system than terrestrial yeasts [152,154]. Okai et al. [151] observed an ethanol concentration 1.4 times higher when they used the salt-tolerant ethanol-producing yeast Zygosaccharomyces rouxii S11. Rattanasaensri et al. [155] isolated three epiphytic yeasts (Candida parapsilosis, Candida glabrata, Kodamaea ohmeri) from the algae Gracilaria fisheri and evaluated the production of ethanol using the same algae as raw material. The authors found that all yeasts showed a high capacity to ferment galactose, with an efficiency of up to 89.6% for C. parapsilosis, important for the fermentation of seaweed.
The evolutionary properties and the adaptive pressure of marine microorganisms remain little explored. Still, great efforts are being made to evaluate the capacity of salt tolerance, hyperthermostability, and cold adaptability. In addition, the production of enzymes that can have new chemical properties with the potential to increase the efficiency of bioprocesses, such as ethanol production, has been evaluated [142,156,157].
The exploitation of yeasts isolated from marine sources is a biofuel production alternative. In saline habitats, it is possible to find a multitude of microorganisms with excellent potential for adaptation to high salt concentrations, capacity for the metabolism of specific sugars, and high osmotic tolerance [133]. Evolutionary engineering is also an interesting alternative for evaluating yeasts used in commercial-scale fermentation vats, considering that the strength of these strains by exposure can improve their fermentative capacity by natural selection of the environment, which may result in yeasts being more tolerant to environmental changes [148,158].
Tolerance to salts is a relevant, but not exclusive, feature for potential yeast strains to be used in seawater-based systems. The biomass hydrolysates fermentation process requires microorganisms to carry out this process efficiently, fermenting different carbon sources — hexoses and pentoses—at the same time. This factor also limits the economic and technological performance of third-generation ethanol production and should be considered in evaluations of potential yeasts [160].
Genetic engineering in microorganisms to increase salt tolerance and efficiently ferment different sugars (e.g., glucose, xylose, fructose, and galactose) is also a promising alternative. However, barriers to feasibility for use in commercial purposes remain. In the study by Casey et al. [153], the addition of chloride, sulfate, potassium, ammonium, and sodium salts in low concentrations (> 0.2 M) had a positive impact on yeast S. cerevisiae 424A (LNH-ST), a yeast strain capable of effectively fermenting both glucose and xylose. The presence of salts increased glucose consumption rates. However, the salts' effects were negative in the xylose conversion into ethanol, regardless of the concentration used. Chloride had the highest potential for inhibition, and there was a change in the metabolic pathway for glycerol production due to the presence of salts. The effects of salt inhibition in recombinant yeasts for co-fermentation of glucose and xylose are influenced by the parent strain used for its development, as Daran-Lapujade et al. [161] observed with the yeast S. cerevisiae CEN.PK113-7D, which demonstrated extreme sensitivity to Na+. The authors suggest the acute sensitivity of the strain CEN.PK might result from a previously-low copy number of ENA genes and, consequently, less transport capacity of cations mediated by Ena6p across the plasma membrane.
Despite the challenges, the possibility of genetically engineering yeasts capable of metabolizing different carbon sources with high fermentation efficiency in systems with high osmotic pressure is an excellent alternative. However, it would be a considerable challenge for the viability of processes based on the use of seawater for ethanol production. Expanding the scale of fermentative processes is always challenging. One of the major problems with the industrial-scale application of seawater is the possibility of corrosion of pipes and vats due to the salt content. This issue could be overcome by covering the entire system with a more resistant material, such as steel or polyvinyl [147].
The more sustainable approach to advancing the use of high-saline-content water and developing yeasts with high capacity to convert different carbon sources into ethanol will trigger a series of improvements in the sector. Biorefineries based on raw materials from marine environments may soon be considered, especially if the industrial facilities are located in coastal regions, facilitating access to seawater, microorganisms, and algae biomass, making the 3G ethanol process viable and with low water and carbon footprint [144,145,147,162,163].
5. Mass and energy balances
This section presents mass and energy balances for bioethanol production from microalgae biomass. In a typical process (Table 4, Fig. 2), within a biorefinery approach, microalgae biomass is pretreated to obtain an extract [164]. The polysaccharides are then hydrolyzed, the hydrolysate is fermented, and the broth is distilled to obtain bioethanol [165]. The comprehensive energy balance of technological routes for the production of third-generation ethanol is important before the economic evaluation. Microalgae can produce much higher yields as lumped from the second-generation to third-generation biofuel with lower resource inputs than other feedstock [166].
Table 4. Description of streams and equipment presented in the theoretical process flow diagram.
| Sector | Equipment | Description | Stream | Description |
|---|---|---|---|---|
| Cultivation and Hydrolysis (100) | BR101 | Bioreactor (microalgae cultivation) | 1 | Supplementation in the reaction medium |
| 2 | Inoculum | |||
| HT201 | Hydrolysis tank | 3 | Microalgae suspension | |
| 4 | Acid feed | |||
| 5 | Steam input | |||
| 6 | Steam output | |||
| Neutralization and Reaction (200) | NT201 | Neutralization tank | 7 | Hydrolyzed broth |
| 8 | Basic solution | |||
| FE201 | Fermentation tank | 9 | Hydrolyzed and neutralized broth | |
| 10 | Inoculum (Saccharomyces cerevisae) | |||
| Purification (300) | DC301 | Distillation column | 11 | Fermented broth |
| 12 | Residual bioproducts | |||
| 13 | Hot water input | |||
| 14 | Hot water output | |||
| CO301 | Condenser | 15 | Vapor bioethanol | |
| 16 | Cold water inlet stream | |||
| 17 | Cold water output stream | |||
| 18 | Liquid bioethanol |
 Fig. 2. Theoretical process flow diagram for microalgae bioethanol production. Mj = global mass flow (kg/h); Tj = Temperature (°C); mi,j = mass flow for each component i (kg/h); i is the subscript referring to the component: water (1), nutrients (2), microalgae (3), acid (4), fermentable sugars (5), basic solution (6), yeast (7), bioethanol (8), neutral salt (9); j is the subscript referring to the stream (1-18); EP101: equipment 1 in sector 100. The meaning of each stream and the code for piece of each equipment are shown in Table 4.
Fig. 2. Theoretical process flow diagram for microalgae bioethanol production. Mj = global mass flow (kg/h); Tj = Temperature (°C); mi,j = mass flow for each component i (kg/h); i is the subscript referring to the component: water (1), nutrients (2), microalgae (3), acid (4), fermentable sugars (5), basic solution (6), yeast (7), bioethanol (8), neutral salt (9); j is the subscript referring to the stream (1-18); EP101: equipment 1 in sector 100. The meaning of each stream and the code for piece of each equipment are shown in Table 4.